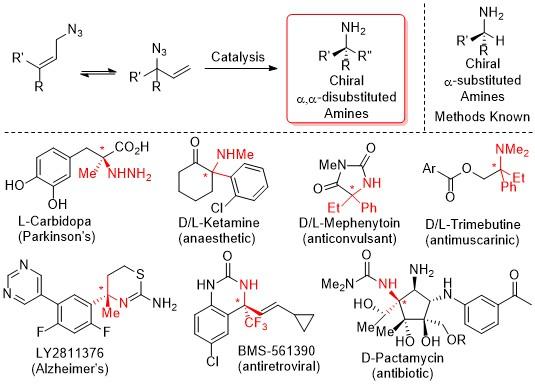
Chiral amines are a fundamental motif present in amino acids, amino sugars, ligands, materials, natural products, and pharmaceuticals. The synthesis of chiral α-substituted amines has been extensively studied and a variety of methods are available for their production. In stark contrast, there is a clear scarcity of methods available for the synthesis of chiral α,α-disubstituted amines. Most of these methods require the covalent attachment of an auxiliary either to attenuate reactivity or selectivity and are thus inherently inefficient. This project aims to prepare chiral α,α-disubstituted amines via direct catalytic methods from readily available feedstocks.
We aim to exploit the curious rearrangement of allylic azides. These compounds readily equilibrate at room temperature and therefore are suitable candidates for dynamic kinetic resolution. These azides can be prepared in a single step from commercial materials. Under selective conditions, they can be converted to single products in very high selectivity. Differentiation of the azide mixture requires extremely selective systems that can manifest enantioselectivity, diastereoselectivity, regioselectivity, site selectivity, and kinetic matching. Developing this selectivity is both challenging and exciting! We are studying the mechanism, limitations, and utility of this process in synthesis.
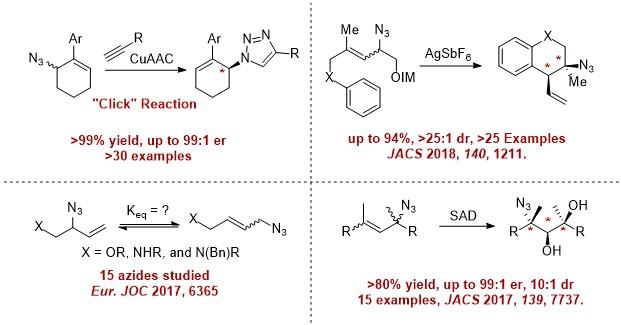
Our lab has proven that this concept is viable with three early examples, and each addresses a unique synthetic challenge. Furthermore, products of these reactions have shown promise as potent and selective GPCR ligands. These ligands connect our fundamental studies on chiral amine synthesis directly to solving societal challenges associated with sustainability and human health. See the publications tab for additional details and developments.